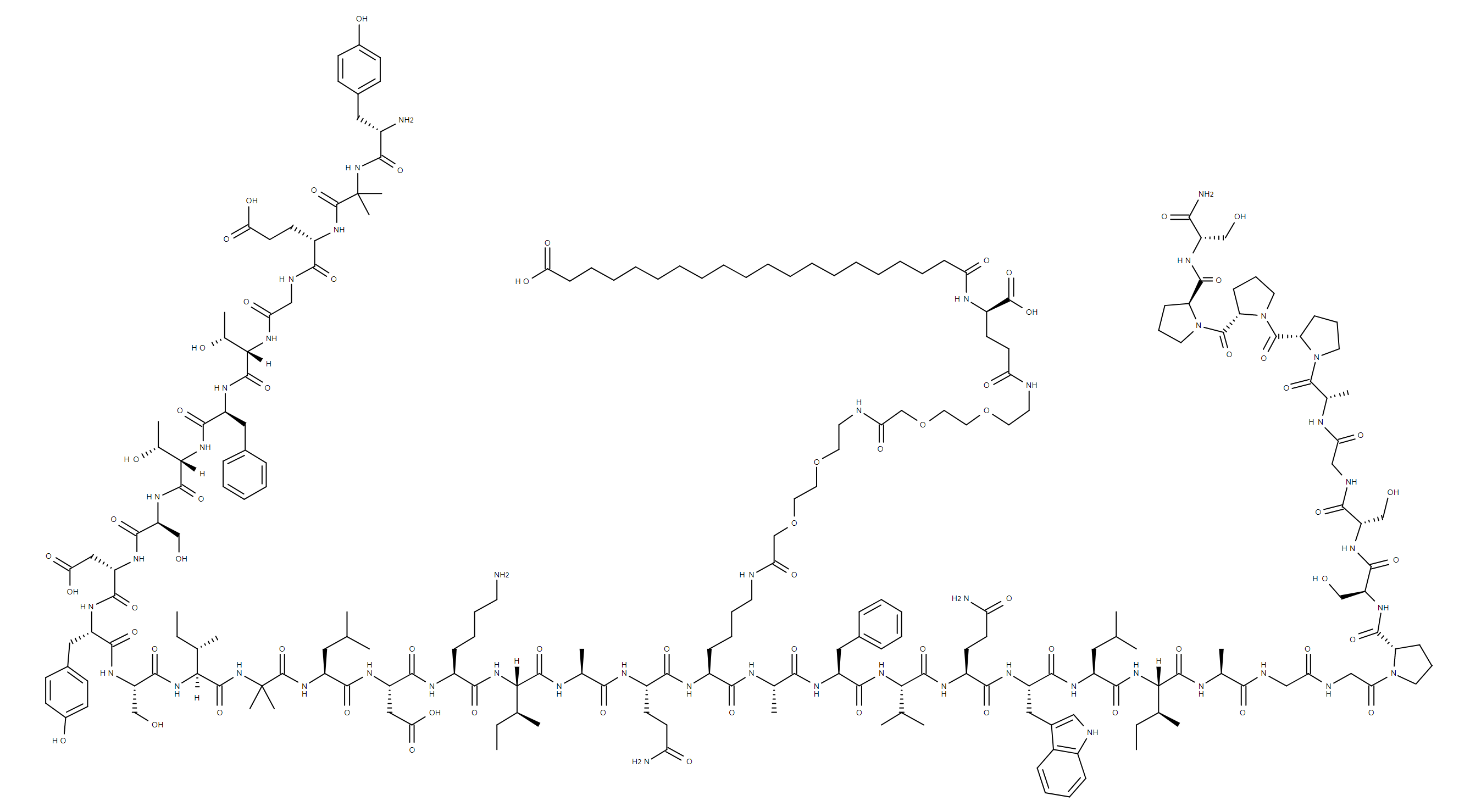
Tirzepatide
Brief Introduction:
Tirzepatide, the CAS number is: 2023788-19-2, which is used with diet and exercise to improve glycemic control in adults with type Ⅱ diabetes.
Specifications of our Tirzepatide:
| Test Items | Specifications |
| Appearance | White or almost white powder |
| Molecular Ion Mass | 4813.45 |
| Purity(HPLC) | Not less than 98.0% |
| Related Substances(HPLC) | Total impurities: ≤2.0% |
| Maximum single impurity: ≤1.0% | |
| Acetic Acid Content(HPLC) | Not more than 15.0% |
| TFA Content(HPLC) | Not more than 1.0% |
| Sodium Ion(IC) | Reported value |
| Water Content(K. F) | Not more than 8.0% |
| pH Value | 7.0 ~ 9.0(C=5mg/mL) |
| Residual Solvents(GC) | Acetonitrile: ≤410 ppm |
| Methanol: ≤3000 ppm | |
| Methylene chloride: ≤600 ppm | |
| Isopropyl ether: ≤5000 ppm | |
| N,N-Dimethylformamide: ≤880 ppm | |
| Solubility | Reported value |
| Oligomer | Not more than 3.0% |
| Bacterial Endotoxins | Not more than 10 EU/mg |
| Microbial Limits | TAMC: ≤500 CFU/g |
| TYMC: ≤500 CFU/g |
Packaging:
1g/bottle, 5g/bottle or according to the specific requirements from customers.
Storage Conditions:
Kept in tight containers, protected from light, stored at a temperature of -20±5℃.
Shelf Life:
24 months after manufacturing date if stored under above mentioned conditions.