Salcaprozate Sodium
Chemical Structural Formula
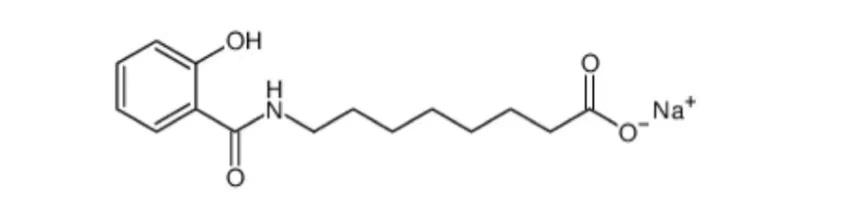
Description
| Testing Items | Specifications | |
| [Characteristic] | Appearance | White to yellowish or pinkish crystalline powder |
| [Identification] | HPLC | The retention time of the main peak of the Sample solution corresponds to that of the Standard solution |
| UV | The maximum absorption should be at the wavelength of 301nm and 238nm, and the minimum absorption should be at the wavelength of 228nm | |
| IR | Corresponding to Cholesterol CRS | |
| [Inspection]
|
Relevant Substances | Impurity A: ≤0.15% |
| Impurity E: ≤0.15% | ||
| Impurity G: ≤0.5% | ||
| The maximum unknown single impurity: ≤0.1% | ||
| Total impurities: ≤1.0% | ||
| Water | ≤2.0% | |
| Heavy Metals | ≤20ppm | |
| Arsenate | ≤0.0002% | |
| Residual Solvents | Methyl Alcohol: ≤0.5% | |
| Isopropanol: ≤0.5% | ||
| DMF: ≤0.088% | ||
| Ethyl Alcohol: ≤0.5% | ||
| Benzene: ≤0.0002% | ||
| Microbial Contamination | TAMC: ≤1000CFU/g | |
| TYMC: ≤100CFU/g | ||
| E. coli should not be detected per 1g | ||
|
Residue on Ignition |
≤0.1%w/w | |
| [Assay] | Sodium | 7.1% ~ 8.1%(On Dried Basis) |
| Salcaprozate Sodium | 98.0% ~ 102.0%(On Dried Basis) | |

Storage Conditions
Kept at 2℃~8℃ under an inert atmosphere.
For reducing the absorption of the moisture, it should be slowly warmed to ambient temperature before opened.
Physical & Chemical Properties
Salcaprozate sodium (SNAC) is an oral absorption enhancer with potential as a delivery agent for oral forms of heparin and insulin. Salcaprozate sodium increases lipophilicity induced by non-covalent macromolecular complexation, thereby increasing passive transcellular penetration of intestinal epithelial cells.
Application
SNAC is an absorption enhancer for dicarbonate phosphate compounds, which is used for treating gastrointestinal diseases, especially for gastrointestinal diseases caused by malabsorption of dicarbonate phosphate compounds.