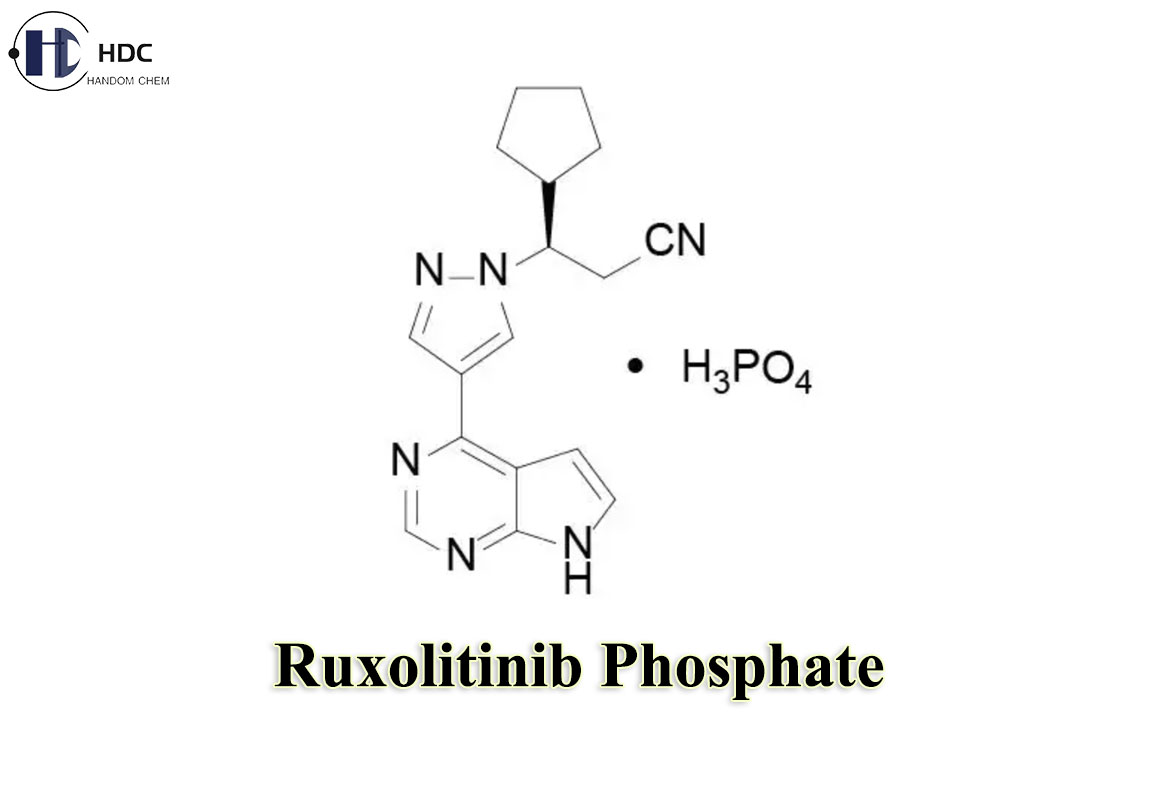
Ruxolitinib Phosphate
Brief Introduction:
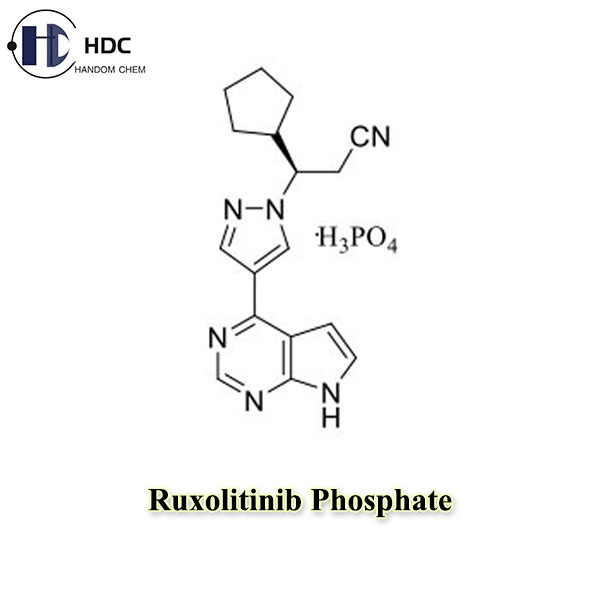
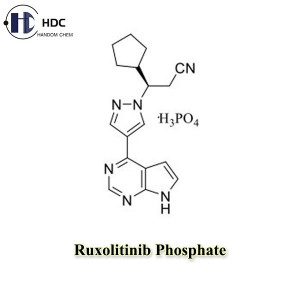
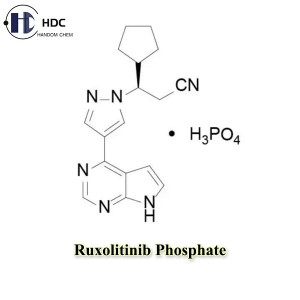
Ruxolitinib Phosphate is the phosphate form of Ruxolitinib, which is a tyrosine kinase inhibitor and is mainly used clinically to treat myeloproliferative neoplasms such as chronic myeloid leukemia and myelofibrosis.
Drug Category:
JAK Inhibitor: Selectively inhibits JAK1 and JAK2 kinases, works by blocking the JAK-STAT signaling pathway, and regulates immune and inflammatory responses.
Mechanism of Action:
Inhibits the abnormally active JAK-STAT pathway, reduces the production of pro-inflammatory cytokines (such as IL-6, IFN-γ), and thus inhibits inflammation and abnormal cell proliferation.
Specifications of our Ruxolitinib Phosphate:
| Test Items | Specifications | |
| Appearance | White to almost white powder | |
| Identification | IR: Identical versus reference spectrum | |
| The retention time of the major peak of the sample solution corresponds to that of the standard solution, as obtained in the assay. | ||
| Water | Not more than 0.5% | |
| pH Value | 2.5 ~ 4.5 | |
| Na | Not more than 100 ppm | |
| Pd | Not more than 10 ppm | |
| PO43- (Anhydrous and no solvent basis) | 22.4% ~ 25.1% | |
| Enantiomer | Impurity A: Not more than 0.7% | |
| Related Substances | Impurity C | Not more than 0.15% |
| Impurity H | Not more than 0.15% | |
| Impurity E | Not more than 0.15% | |
| Impurity D | Not more than 0.15% | |
| Impurity F | Not more than 0.15% | |
| Impurity B | Not more than 0.15% | |
| Individual unspecified impurity | Not more than 0.10% | |
| Total impurities | Not more than 0.5% | |
| Residual Solvents Ⅰ | Methanol | Not more than 3000 ppm |
| Acetone | Not more than 5000 ppm | |
| 2-Propanol | Not more than 5000 ppm | |
| Dichloromethane | Not more than 600 ppm | |
| Tert-Butylmethyl ether | Not more than 5000 ppm | |
| Ethyl acetate | Not more than 5000 ppm | |
| 1,4-Dioxane | Not more than 380 ppm | |
| Residual Solvents Ⅱ | n-Heptane | Not more than 4000 ppm |
| N,N-Dimethylformamide | Not more than 880 ppm | |
| Dimethyl sulfoxide | Not more than 5000 ppm | |
| Residual Solvents Ⅲ | Benzene | Not more than 2 ppm |
| Microbiological Limits | Total aerobic microbial count | Not more than 1000 CFU/g |
| Total combined yeasts & mould count | Not more than 100 CFU/g | |
| Escherichia coli | Negative | |
| Assay (Anhydrous and no solvent basis) | 98.0% ~ 102.0% | |
Indications:
※ Myelofibrosis: Relieve splenomegaly and related symptoms (e.g., pain, fatigue).
※ Polycythemia Vera: For patients resistant or intolerant to hydroxyurea.
※ Atopic Dermatitis: Topical or systemic treatment of moderate to severe cases.
※ Graft Versus Host Disease (GVHD): Steroid-refractory acute GVHD.
Preparation and Usage:
- Oral Tablets or Topical Application (depending on the indication).
♔ The dosage should be adjusted according to the patient's condition.
Packaging:
10g/Bag, 20g/Bag, 50g/Bag, 100g/Bag, 500g/Bag, 1kg/Bag or according to the specific requirements from customers.
Storage Conditions:
Preserved in unopened original containers in a cool dry place before using; kept away from direct sunlight, heat and moisture.
Shelf Life:
36 months from the date of manufacturing when stored under the above conditions.