Refined Soy Lecithin (Pharma Grade)
Brief Introduction to Phospholipids:
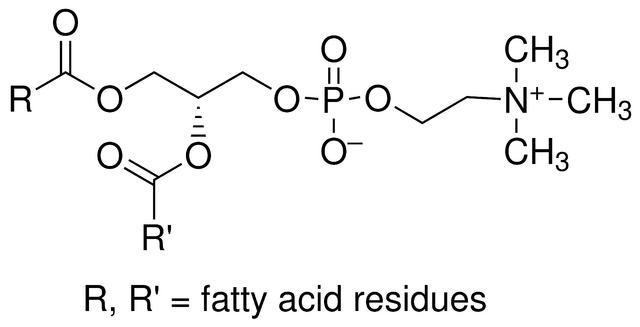
Phospholipids are a type of lipid containing phosphorus, including glycerophospholipids and sphingomyelin. Glycerophospholipids are widely distributed in animals, plants and microorganisms, while sphingomyelins are generally only distributed in animals and microorganisms, and are extremely rare in plants. Commodity phospholipids mostly refer to glycerophospholipids, such as plant phospholipids (soybean phospholipids, rapeseed phospholipids, cottonseed phospholipids, etc.) and egg yolk phospholipids, whose main components are phosphatidylcholine (PC), phosphatidylethanolamine (PE), and phosphatidylinositol (PI).
The chemical name of lecithin is phosphatidylcholine (PC for short), according to its purity, it is generally divided into PC50, PC60, PC70, PC80, PC90, PC95 and other product forms. The highest purity can be purified to 98%, since the higher the purity, the stronger the oxidation performance, the lecithin purified to 98% needs to be hydrogenated before preservation. Lecithin that has not been hydrogenated is generally required to be stored in a sealed container filled with nitrogen.
Pure lecithin is a colorless, odorless, white solid at room temperature. In fact, it appears light yellow to brown due to different preparation or refining methods and storage conditions.
Ordinary Pharmaceutical Grade Soya Lecithin:
Ordinary pharmaceutical grade soybean lecithin (phosphatidylcholine, referred to as PC) is a yellow waxy product made from soybean powder lecithin as raw material, extracted through ethanol and other processes using original patented equipment (ZL2017.20049599.7; ZL2010.20147413.X). The main specifications include PC-50 and PC-70.
Product Specifications:
| Physical and Chemical Indicators | ||
| PC-50 | PC-70 | |
| Appearance | Yellow waxy solid | |
| Phosphorus (P) | ≥2.7% | ≥2.7% |
| Nitrogen (N) | 1.5%~2.0% | 1.5%~2.0% |
| Phosphatidylcholine (PC) | ≥45.0% | ≥70.0% |
| Phosphatidylethanolamine (PE) | ≤30.0% | ≤30.0% |
| Phosphatidylcholine (PC) + Phosphatidylethanolamine (PE) | ≥70% | ≥70% |
| Acetone Insolubles | ≥90.0% | ≥90.0% |
| Hexane Insolubles | ≤0.3% | ≤0.3% |
| Moisture | ≤1.5% | ≤1.5% |
| Total Residual Solvents | ≤0.5% | ≤0.5% |
| Acid Value | ≤30mgKOH/g | ≤30mgKOH/g |
| Iodine Value | ≥75gI/100g | ≥75gI/100g |
| Peroxide Value | ≤3.0 | ≤3.0 |
| Microorganisms | ||
| PC-50 | PC-70 | |
| Total Aerobic Bacteria Count | ≤100CFU/g | ≤100CFU/g |
| Total combined Yeasts & Mould Count | ≤100CFU/g | ≤100CFU/g |
| Escherichia coli | Negative/g | Negative/g |
| Salmonella | Negative/10g | Negative/10g |
| Heavy Metals | ||
| PC-50 | PC-70 | |
| Total Heavy Metals | ≤20ppm | ≤20ppm |
| Arsenic (As) | ≤2ppm | ≤2ppm |
| Lead (Pb) | ≤2ppm | ≤2ppm |
Scope of Application:
| Medical Products | Pharmaceutical grade soya lecithin is widely used as an emulsifier, solubilizer, dispersant for oral preparations, as well as a bioavailability enhancer and local penetration enhancer for oral drugs. |
Pharmaceutical Grade Soya Lecithin (for injection):
Pharmaceutical grade soya lecithin (for injection) is a pale yellow waxy product made from powdered lecithin through ethanol extraction and other processes using original patented equipment (ZL2017.20049599.7; ZL2010.20147413.X). It has good emulsification, wettability, viscosity adjustment, dispersibility, antioxidant properties and its own unique physiological activity, and is widely used in the fields of emulsions for injection, raw materials for liposomes and fluorocarbon emulsions.
Product Specifications:
| Physical and Chemical Indicators | ||||
| PC-80 | PC-90 | PC-95 | PC-98 | |
| Appearance | Yellow waxy solid | |||
| Phosphorus (P) | ≥2.7% | ≥2.7% | ≥2.7% | ≥2.7% |
| Nitrogen (N) | 1.5%~2.0% | 1.5%~2.0% | 1.5%~2.0% | 1.5%~2.0% |
| Phosphatidylcholine (PC) | ≥80% | ≥90% | ≥95% | ≥98% |
| Phosphatidylethanolamine (PE) | ≤30% | ≤30% | ≤30% | ≤30% |
| Phosphatidylcholine (PC) + Phosphatidylethanolamine (PE) | ≥80% | ≥90% | ≥95% | ≥98% |
| Acetone Insolubles | ≥90% | ≥90% | ≥90% | ≥90% |
| Lysophosphatidylethanolamine (LPE) | ≤1% | ≤1% | ≤1% | ≤1% |
| Lysophosphatidylcholine (LPC) | ≤3.5% | ≤3.5% | ≤3.5% | ≤3.5% |
| Lysophosphatidylethanolamine (LPE) + Lysophosphatidylcholine (LPC) | ≤4.0% | ≤4.0% | ≤4.0% | ≤4.0% |
| Phosphatidylinositol (PI) | ≤5.0% | ≤5.0% | ≤5.0% | ≤5.0% |
| Total Related Substances | ≤8.0% | ≤8.0% | ≤8.0% | ≤8.0% |
| Hexane Insolubles | ≤0.3% | ≤0.3% | ≤0.3% | ≤0.3% |
| Moisture | ≤1.5% | ≤1.5% | ≤1.5% | ≤1.5% |
| Total Residual Solvents | ≤0.5% | ≤0.5% | ≤0.5% | ≤0.5% |
| Protein | Should not be detected | Should not be detected | Should not be detected | Should not be detected |
| Acid Value | ≤30mgKOH/g | ≤30mgKOH/g | ≤30mgKOH/g | ≤30mgKOH/g |
| Iodine Value | ≥75gI/100g | ≥75gI/100g | ≥75gI/100g | ≥75gI/100g |
| Peroxide Value | ≤3.0 | ≤3.0 | ≤3.0 | ≤3.0 |
| Microorganisms, bacterial endotoxins | ||||
| PC-80 | PC-90 | PC-95 | PC-98 | |
| Total Aerobic Bacteria Count | ≤100CFU/g | ≤100CFU/g | ≤100CFU/g | ≤100CFU/g |
| Total combined Yeasts & Mould Count | ≤100CFU/g | ≤100CFU/g | ≤100CFU/g | ≤100CFU/g |
| Escherichia coli | Negative/g | Negative/g | Negative/g | Negative/g |
| Salmonella | Negative/10g | Negative/10g | Negative/10g | Negative/10g |
| Endotoxins | <2.0EU/g | <2.0EU/g | <2.0EU/g | <2.0EU/g |
| Heavy Metals | ||||
| PC-80 | PC-90 | PC-95 | PC-98 | |
| Total Heavy Metals | ≤5ppm | ≤5ppm | ≤5ppm | ≤5ppm |
| Arsenic (As) | ≤2ppm | ≤2ppm | ≤2ppm | ≤2ppm |
| Lead (Pb) | ≤2ppm | ≤2ppm | ≤2ppm | ≤2ppm |
Other specifications of our Pharmaceutical Grade Soya Lecithin(for injection) can also be customized according to the specific needs of different customers.
Scope of Application:
| Medical Products | Our pharmaceutical grade soybean lecithin (for injection) is widely used in the fields of injection preparations, liposome raw materials and fluorocarbon emulsions. |
Packaging of our Pharmaceutical Grade Soya Lecithin:
1) Sizes:
① Small Ones: 10g/bag, 20g/bag, 50g/bag, 100g/bag or 500g/bag.
② Larger Ones: 5kg/Carton(1kg/Bag×5 Bags), 10kg/Carton(1kg/Bag×10 Bags or 5kg/Bag×2 Bags).
2) Packaging Materials:
① Inner Packaging: The inner packaging adopts double-layer packaging: the inner layer is a medicinal low-density polyethylene bag, and the outer layer is an aluminum foil bag;
② Outer Packaging: The outer packaging adopts double-layer packaging: the inner layer is a foam box with a built-in ice box and the outer layer is a carton.
Storage Conditions:
Store in an unopened container, away from light and at low temperature (below -18°C).
Shelf Life:
12 months from the date of manufacturing when stored under the above conditions.