Tirzepatide (5mg/10mg/15mg/20mg/30mg)

간단한 소개 :
Tirzepatide는 세계 최초이자 현재 유일한 포도당 의존성 인슐린 폴리펩티드 (GIP)/글루카곤 유사 펩티드 -1 (GLP-1) 수용체 작용제입니다. Eli Lilly가 개발 한이 회사는 2024 년 5 월 21 일 중국에서 마케팅을 승인했으며 2025 년 1 월 2 일에 공식적으로 출시되었습니다.식이 제어 및 운동에 기초하여 메트포르민 및/또는 설 포닐 우레아 약물에 의해 여전히 혈당이 제어되지 않는 유형의 당뇨병 환자에게 적합합니다.
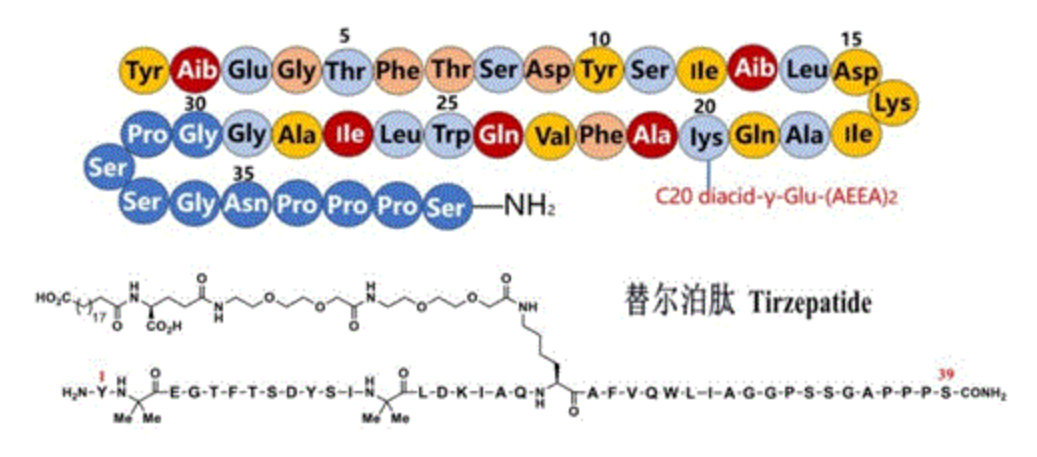
행동 메커니즘 :
Tirzepatide는 2 개의 인크 레틴 호르몬 GIP 및 GLP-1의 효과를 하나의 분자에 통합하고 동시에 이들 두 펩티드 호르몬의 수용체를 활성화시켜 더 많은 임상 효과를 제공합니다. 그 중에서도, GLP-1 수용체의 활성화는 위 배출을 지연시키고, 식욕을 감소시키고, 혈당을 낮추며, 인슐린 감수성을 향상시킨다; GIP 수용체의 활성화는 인슐린 분비를 향상시키고 글루카곤 방출을 감소시키고 지방 대사를 촉진합니다.
임상 시험 및 효능 :
Surpass라는 일련의 임상 시험에서, Tirzepatide는 우수한 효능을 나타냈다. 유형 ⅱ 당뇨병 환자의 치료에서, 티르 제 페타이드로 단일 요법 후, 환자에서 당화 헤모글로빈 (HBA1C)의 평균 감소는 2.0% ~ 2.5%에 도달했다. 그 중에서도, 고용량 (15mg) 티르 제파 시드 그룹은 1mg semaglutide 그룹 (HBA1C 감소 vs 2.5%)보다 유의하게 더 우수했다.
Tirzepatide는 또한 체중 감량에 뛰어난 효과를 나타 냈습니다. Surmount 시험 (비만 또는 과체중 비 당뇨병 환자의 경우)에서, 고용량 (15mg) 그룹에서 환자의 평균 체중 감소는 20.9%였으며, 체중 감소는 약 22.5 kg이고; 중간 용량 (10mg) 그룹의 체중 감소는 19.5%였으며 체중 감소는 약 20.9kg입니다. 이 중요한 체중 감량 효과는 Tirzepatide를 체중 감량 분야에서 뜨거운 주제로 만들었습니다.
또한, Tirzepatide는 또한 더 많은 전신 이점을 가질 수 있습니다. 예를 들어, 심부전 퇴치 및 심혈관 사망률 감소 측면에서, 티르 제자 타이드는 또한 큰 장점과 잠재력을 보여 주었다. 인슐린 저항성을 감소시키고 심근 대사를 향상시킬 수 있으며, 심근을 보호 할 수있는 항 염증 및 항 산화 스트레스 효과가 있습니다. 체중 감량을 촉진하고 혈압을 낮추는 데 티르 제 페이드의 효과는 심장의 부담을 줄입니다.
비디오 1 :
비디오 2 :
체중 감량 메커니즘 :
Tirzepatide의 체중 감량 메커니즘은 주로 GIP 및 GLP-1 수용체의 활성화와 관련이 있습니다. 이들 수용체를 활성화함으로써, 티르 제제 타이드는 식욕을 줄이고, 위 배출을 지연시키고, 지방 대사를 촉진시켜 체중 감량의 영향을 달성 할 수있다. 또한, Tirzepatide는 또한 인슐린 감수성을 향상시키고 체중을 더욱 감소시킬 수 있습니다.
사용 및 복용량 :
Tirzepatide는 유형 ⅱ 당뇨병 및 체중 감소의 치료를 위해 일주일에 한 번 주사되는 주간 준비입니다. 이중 메커니즘은 혈당 및 체중 조절에 대한 요구 사항이 높은 환자에게 이상적인 선택입니다.

Tirzepatide (5mg/vial)의 사양 :
| 테스트 항목 | 명세서 | |
| 모습 | 흰색 또는 거의 흰색 가루 | |
| 용해도 | 물에 자유롭게 용해됩니다 | |
| HPLC에 의한 식별 | 시험 솔루션의 주요 피크의 체류 시간은 분석에서 얻은 바와 같이 참조 용액의 유지 시간에 해당합니다. | |
| MS에 의한 분자 이온 질량 | 4813.45 ± 1.0 | |
| 아미노산 함량 | ASP | 1.6 ~ 2.4 |
| Tyr | 1.6 ~ 2.4 | |
| lys | 1.6 ~ 2.4 | |
| 일 | 2.0 ~ 3.2 | |
| 레 | 1.6 ~ 2.4 | |
| 발 | 0.8 ~ 1.2 | |
| thr | 1.6 ~ 2.4 | |
| Phe | 1.6 ~ 2.4 | |
| Ser | 4.0 ~ 6.0 | |
| 알라 | 3.2 ~ 4.8 | |
| 글리 | 3.2 ~ 4.8 | |
| 글루 | 3.2 ~ 4.8 | |
| 찬성 | 3.2 ~ 4.8 | |
| AIB | N/A | |
| AEEA | N/A | |
| 수분 함량 (K. F) | 8.0% 이상 | |
| 솔루션 선명도와 색상 | 명확하고 무색 | |
| 순도 (HPLC) | 99.0% 이상 | |
| 관련 물질 (HPLC) | 총 불순물 | 1.0% 이하 |
| 최대 단일 불순물 | 0.5% 이하 | |
| 분자 덩어리가 티르 제이퍼 사이드보다 큰 불순물 (크기 제외) | 0.50% 이하 | |
| 잔류 용매 | 아세토 니트릴 | 410 ppm 이하 |
| 메탄올 | 3000 ppm 이하 | |
| 박테리아 내 독소 | 10 EU/mg 미만 | |
| 미생물 한계 | TAMC | 100 CFU/g를 넘지 않습니다 |
| TYMC | 100 CFU/g를 넘지 않습니다 | |
| 트리 플루오로 아세테이트 이온 | 0.10% 이하 | |
| 나트륨 이온 | 5.0% 이하 | |
| 아세테이트 이온 | 0.10% 이하 | |
| 인산염 이온 | 0.10% 이하 | |
| 펩티드 함량 | 5.0 mg 이상 | |
| 분석 (HPLC) | 95.0% ~ 105.0% (무수 및 소금이없는 물질 기준) | |
Tirzepatide (15mg/vial)의 사양 :
| 테스트 항목 | 명세서 | |
| 모습 | 흰색 또는 거의 흰색 가루 | |
| 용해도 | 물에 자유롭게 용해됩니다 | |
| HPLC에 의한 식별 | 시험 솔루션의 주요 피크의 체류 시간은 분석에서 얻은 바와 같이 참조 용액의 유지 시간에 해당합니다. | |
| MS에 의한 분자 이온 질량 | 4813.45 ± 1.0 | |
| 아미노산 함량 | ASP | 1.6 ~ 2.4 |
| Tyr | 1.6 ~ 2.4 | |
| lys | 1.6 ~ 2.4 | |
| 일 | 2.0 ~ 3.2 | |
| 레 | 1.6 ~ 2.4 | |
| 발 | 0.8 ~ 1.2 | |
| thr | 1.6 ~ 2.4 | |
| Phe | 1.6 ~ 2.4 | |
| Ser | 4.0 ~ 6.0 | |
| 알라 | 3.2 ~ 4.8 | |
| 글리 | 3.2 ~ 4.8 | |
| 글루 | 3.2 ~ 4.8 | |
| 찬성 | 3.2 ~ 4.8 | |
| AIB | N/A | |
| AEEA | N/A | |
| 수분 함량 (K. F) | 8.0% 이상 | |
| 솔루션 선명도와 색상 | 명확하고 무색 | |
| 순도 (HPLC) | 99.0% 이상 | |
| 관련 물질 (HPLC) | 총 불순물 | 1.0% 이하 |
| 최대 단일 불순물 | 0.5% 이하 | |
| 분자 덩어리가 티르 제이퍼 사이드보다 큰 불순물 (크기 제외) | 0.50% 이하 | |
| 잔류 용매 | 아세토 니트릴 | 410 ppm 이하 |
| 메탄올 | 3000 ppm 이하 | |
| 박테리아 내 독소 | 10 EU/mg 미만 | |
| 미생물 한계 | TAMC | 100 CFU/g를 넘지 않습니다 |
| TYMC | 100 CFU/g를 넘지 않습니다 | |
| 트리 플루오로 아세테이트 이온 | 0.10% 이하 | |
| 나트륨 이온 | 5.0% 이하 | |
| 아세테이트 이온 | 0.10% 이하 | |
| 인산염 이온 | 0.10% 이하 | |
| 펩티드 함량 | 15.0 mg 이상 | |
| 분석 (HPLC) | 95.0% ~ 105.0% (무수 및 소금이없는 물질 기준) | |
Tirzepatide (60mg/vial)의 사양 :
| 테스트 항목 | 명세서 | |
| 모습 | 흰색 또는 거의 흰색 가루 | |
| 용해도 | 물에 자유롭게 용해됩니다 | |
| HPLC에 의한 식별 | 시험 솔루션의 주요 피크의 체류 시간은 분석에서 얻은 바와 같이 참조 용액의 유지 시간에 해당합니다. | |
| MS에 의한 분자 이온 질량 | 4813.45 ± 1.0 | |
| 아미노산 함량 | ASP | 1.6 ~ 2.4 |
| Tyr | 1.6 ~ 2.4 | |
| lys | 1.6 ~ 2.4 | |
| 일 | 2.0 ~ 3.2 | |
| 레 | 1.6 ~ 2.4 | |
| 발 | 0.8 ~ 1.2 | |
| thr | 1.6 ~ 2.4 | |
| Phe | 1.6 ~ 2.4 | |
| Ser | 4.0 ~ 6.0 | |
| 알라 | 3.2 ~ 4.8 | |
| 글리 | 3.2 ~ 4.8 | |
| 글루 | 3.2 ~ 4.8 | |
| 찬성 | 3.2 ~ 4.8 | |
| AIB | N/A | |
| AEEA | N/A | |
| 수분 함량 (K. F) | 8.0% 이상 | |
| 솔루션 선명도와 색상 | 명확하고 무색 | |
| 순도 (HPLC) | 99.0% 이상 | |
| 관련 물질 (HPLC) | 총 불순물 | 1.0% 이하 |
| 최대 단일 불순물 | 0.5% 이하 | |
| 분자 덩어리가 티르 제이퍼 사이드보다 큰 불순물 (크기 제외) | 0.50% 이하 | |
| 잔류 용매 | 아세토 니트릴 | 410 ppm 이하 |
| 메탄올 | 3000 ppm 이하 | |
| 박테리아 내 독소 | 10 EU/mg 미만 | |
| 미생물 한계 | TAMC | 100 CFU/g를 넘지 않습니다 |
| TYMC | 100 CFU/g를 넘지 않습니다 | |
| 트리 플루오로 아세테이트 이온 | 0.10% 이하 | |
| 나트륨 이온 | 5.0% 이하 | |
| 아세테이트 이온 | 0.10% 이하 | |
| 인산염 이온 | 0.10% 이하 | |
| 펩티드 함량 | 60.0 mg 이상 | |
| 분석 (HPLC) | 95.0% ~ 105.0% (무수 및 소금이없는 물질 기준) | |
실험실 및 장비 디스플레이 :

운송 모드 :

결제 방법 :

저장 조건 :
조명으로부터 보호되는 도구 단단한 용기에 보존됩니다.
(권장 온도 : -25 ℃ ~ -15 ℃)
유령 수명 :
상기 조건 하에서 저장 될 때 제조 일로부터 24 개월.