Betadex sulfobutyl ether sodium
Chemical Structural Formula
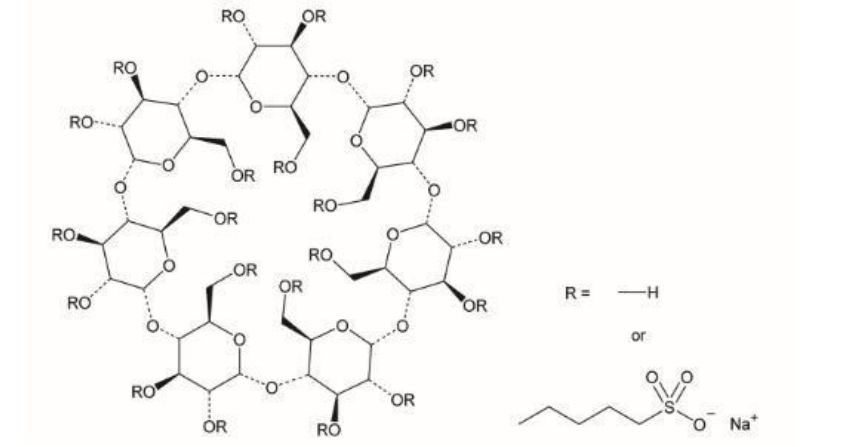
Description
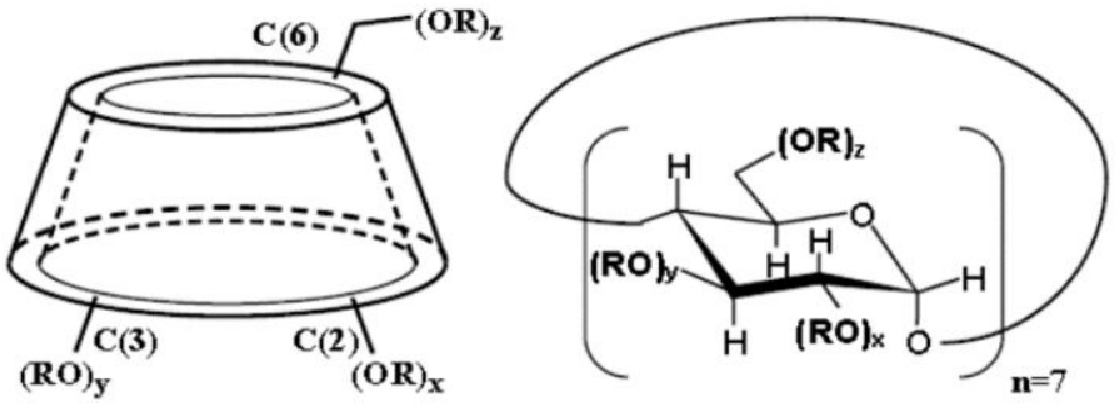
The betadex sulfobutyl ether sodium produced by HDC CHEM is the product of substitution reaction between β-cyclodextrin and 1,4-butane sultone. The glucose unit of beta cyclodextrin is formed by linking 7 pyranose through a-1, 4 glycosidic bonds. The substitution reaction occurs at the 2, 3, and 6 carbon hydroxyl positions of the β-CD glucose unit, and the obtained product is a substitution SBECD with a degree of 6.2-6.9, the structure diagram is as follows:
| [Product Features] | It can be used as solubilizer, wetting agent, chelating agent (complexing agent), multivalent masking agent. Sodium sulfobutylbeta cyclodextrin is a new type of anionic highly water-soluble cyclodextrin derivative, which can be well included with drug molecules to form non-covalent complexes, thereby improving the stability, water solubility and safety of drugs. It can reduce nephrotoxicity, alleviate drug hemolysis, control drug release rate, mask bad smell, etc. It has good solubilization, convenient drug administration, safety and stability.Compared with beta-cyclodextrin (β-CD), Betadex sulfobutyl ether sodium has better water solubility, less hemolysis, and lower nephrotoxicity. It is a new type of medicinal drug with very broad application prospects Accessories. |
|
[Product Performance] |
1. Solubilization:Neutral, positive and negative drug substances can be effectively combined with Sulfobutyl Ether-β-cyclodextrin, so that compounds with different solubility in the drug substance can be increased by 10 to 25000 times.
2. Convenient Drug Administration: Sulfobutyl Ether-β-cyclodextrin has good biocompatibility and can be administered by injection, oral, ophthalmic, nasal, topical and inhalation. 3. Good Security: It is generally rapidly and completely eliminated from the kidneys after administration. In vitro experiments and in vivo acute, subacute and chronic toxicity studies provide safety data and are approved for human pharmaceutical formulations. 4. Good Stability: The interaction with sulfobutylbeta cyclodextrin can provide a beneficial protective environment for the drug substance in its lipophilic cavity, while the hydrophilic surface provides excellent water solubility to provide solubility and stability. |
|
[Application Cases] |
Betadex sulfobutyl ether sodium is often used in insoluble compounds, such as Voriconazole, Carfilzomib, Ziprasidone, Aripiprazole, Maropitant (Animal Medicine), Posaconazole, Carbamazepine, Melphalan, Delafloxacin, Mebendazole, Topiramate, Omeprazole, Clopidogrel, Docetaxel, Sofosbuvir, Ziprasidone Mesylate, Meloxicam, Tetrahydroprogesterone, and several other nitrogenous API bases are in various clinical stages. |
|
[Specific Applications In The Products]
|
1. Application in Injection(1) Function: Solubilizer, stabilizer, improve the solubility and stability of insoluble drugs, and make insoluble drugs developed into injections.
(2) Example: Use betadex sulfobutyl ether sodium to improve the solubility of Voriconazole, Posaconazole, Delafloxacin, Docetaxel, Ibuprofen and Indomethacin; Using betadex sulfobutyl ether sodium to improve the stability of Carmustine. |
| 2. Application in Oral Preparations(1) Function: Solubilizer, stabilizer, improve the bioavailability of insoluble drugs.
(2) Example: Improve the bioavailability of Flunarizine, Danazol, Prednisolone and Prasugrel with Sulfobutyl Ether-β-cyclodextrin. |
|
| 3. Application in Ophthalmic Preparations(1) Function: Solubilizer, stabilizer, reduce drug irritation.
(2) Example: Use Sulfobutyl Ether-β-cyclodextrin to improve the irritation and stability of Pilocarpine, Dipivefrin, Balofloxacin and Ganciclovir. |
|
| 4. Application in Nasal Preparations(1) Function: Increase the permeability of the nasal mucosa, improve the solubility and stability of the drug, and improve the metabolic rate of the target drug.
(2) Example: Using Sulfobutyl Ether-β-cyclodextrin to improve the solubility and stability of Midazolam. |
|
| 5. Ointment(1) Function: Improve the solubility and stability of drugs.
(2) Example: Using betadex sulfobutyl ether sodium to improve the solubility and stability of Nimesulide. |

| Testing Items | Specifications | |
| Appearance | White to off-white amorphous powder | |
| Solubility | Very soluble in water, sparingly soluble in Methanol, practically insoluble in Ethanol, N-Hexane, 1-Butanol, Acetonitrile, 2-Propanol and Ethyl Acetate. | |
|
Identification |
IR | Same absorption bands as USP betadex sulfobutyl ether sodium RS. |
| HPLC | The retention time of the major peak of Sample solution corresponds to that of the Standard solution. | |
| Average degree of substitution | Conforms | |
| Sodium | Identify tests are positive for Sodium | |
| Assay(On Dried Bsis) | 95.0% ~ 105.0% | |
| Betadex | Not more than 0.1% | |
| 1,4-Butane Sultone | Not more than 0.5ppm | |
| Sodium Chloride | Not more than 0.2% | |
| 4-Hydroxybutane-1-Sulfonic Acid | Not more than 0.09% | |
| Bis(4-Sulfobutyl) Ether Disodium | Not more than 0.05% | |
| Bacterial Endotoxins | Not more than 0.02EU/mg | |
| The Total Aerobic Microbial Counts | Not more than 100CFU/g | |
| The Total Combined Moulds and Yeasts Counts | Not more than 50CFU/g | |
| Escherichia coli | Negative | |
| Clarity of Solution | 30%(w/v) solution is clear and essentially free from particles of foreign matters. | |
| Average Degree of Substitution | 6.2 ~ 6.9 | |
|
Peaks Ⅰ-Ⅹ(% peak area) |
Ⅰ |
0.0 ~ 0.3 |
|
Ⅱ |
0.0 ~ 0.9 | |
|
Ⅲ |
0.5 ~ 5.0 | |
|
Ⅳ |
2.0 ~ 10.0 | |
|
Ⅴ |
10.0 ~ 20.0 | |
|
Ⅵ |
15.0 ~ 25.0 | |
|
Ⅶ |
20.0 ~ 30.0 | |
|
Ⅷ |
10.0 ~ 25.0 | |
|
Ⅸ |
2.0 ~ 12.0 | |
|
Ⅹ |
0.0 ~ 4.0 | |
| pH Value | 4.0 ~ 6.8 | |
| Water Determination | Not more than 10.0% | |
Packaging
1) Inner Packing: Sterile PE Bag + Aluminum Foil Bag;
2) Outer Packing: Cardboard Drum(Fibre Drum) or Carton.
3) Packing Specification(Injection Grade): 10kg/drum or 20kg/drum.
Storage Conditions
Preserved in unopened original containers in a cool dry place before using; kept away from direct sunlight, heat and moisture.
Shelf Life
24 months if stored under above mentioned conditions.










